RESEARCH – Mechanisms of stress adaptation
Stress is part of our daily life and usually we are well set to cope with adverse events. When stress exposure is prolonged or too intense, psychopathologies like depression or anxiety disorders can develop. However, the level of stress required for such a mal-adaptation is highly individual. Even after traumatic, potentially life-threatening stress like car accidents, natural disasters or terror attacks most of the exposed people will recover, while a minority becomes lastingly affected and may develop anxiety disorders such as posttraumatic stress disorder (PTSD).
GABAergic mechanisms
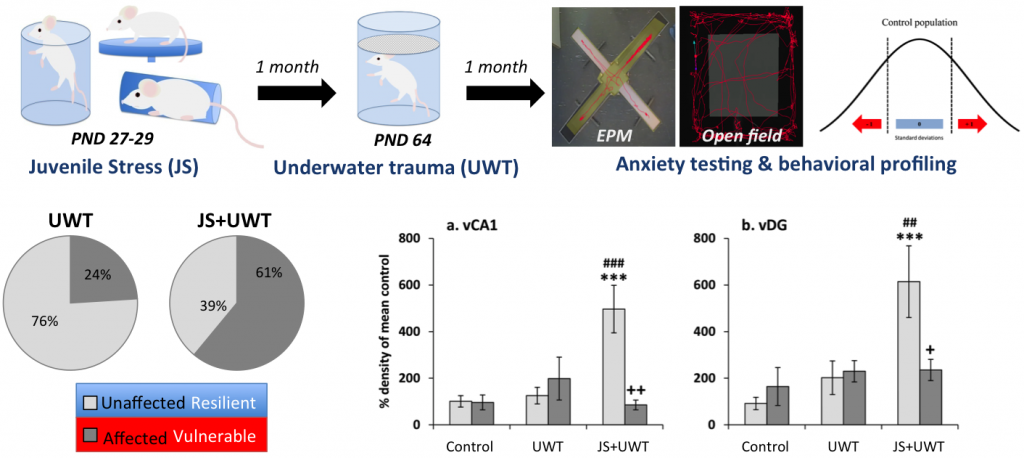
In our research we try to understand which neuronal mechanism support a successful adaptation even to traumatic stress and which contribute to stress pathology. To that end we employ different stress exposure models in mice and rats (in collaboration with Gal Richter-Levin, University of Haifa). By subsequent behavioral profiling we can identify lastingly affected, vulnerable vs. resilient, unaffected animals. With this approach we could already demonstrate that risk factors like stress during the juvenile life phase enhances the prevalence for mal-adaptation to traumatic stress later in life and changes the expression profile of GABA receptor subunits in the ventral hippocampus (Ardi et al., 2016).
Next to behaviorally induced stress, we also work with genetic models for increased stress susceptibility. In Michelle Ulrich‘s research project we investigate the emotional and cognitive responses of mice lacking the gene for the GABA synthetizing enzyme GAD65. These mice express heightened levels of anxiety and altered fear memory. Our current work focuses on sex differences in emotion and cognition responses. Together with Evangelia Pollali and Gürsel Çalışkan from the Institute of Biology at the Otto-von-Guericke University we further investigate oscillatory activity within the limbic system as correlates for altered anxiety responses.
Neuropeptides & stress resilience
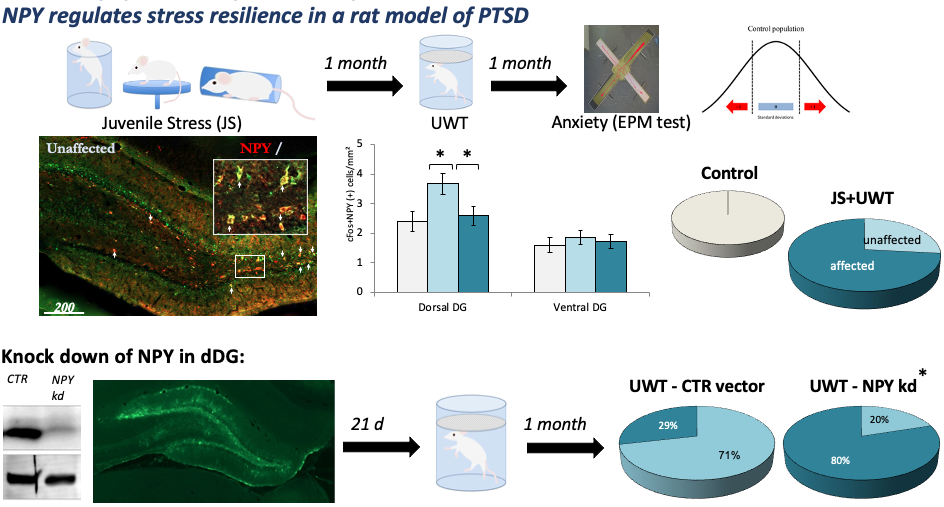
Many GABAergic neurons, especially in the hippocampus, use neuropeptides as co-transmitters that can regulate neuronal function. Neuropeptide Y has been identified as a resilience enhancing agent in various animal models of stress as well as in patients with stress-induced psychopathologies. In collaboration with Stav Regev-Tsur and Gal Richter-Levin from the University of Haifa we found that NPY within the dorsal dentate gyrus promotes resilience in the juvenile stress + underwater trauma model of Posttraumatic Stress disorder in rats (Regev-Tsur et al., 2020). This was achieved by a local knock down of NPY in this brain region and emphasized the importance of the dentate gyrus in mediating emotion.
Astroglia
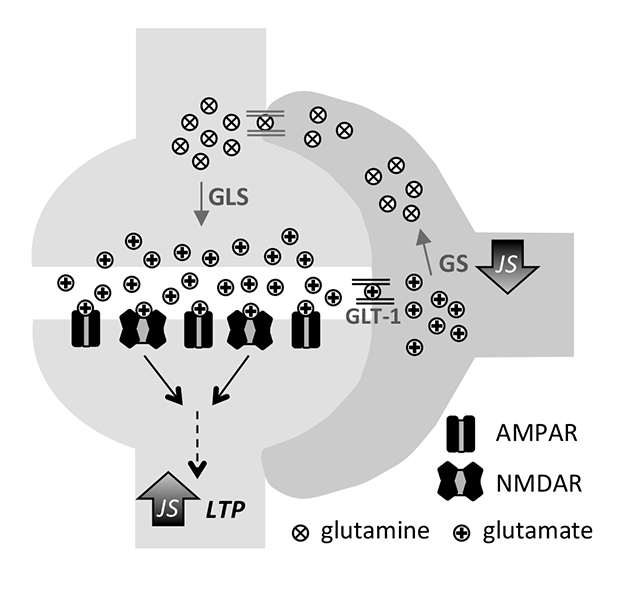
In recent years interest in glia cells grew because of their ability in lastingly modulate neurotransmission and neuronal metabolism.
Using juvenile stress as a model for childhood adversity, we could show that GABA uptake by astrocytes is lastingly reduced within the dorsal dentate gyrus, resulting in shifted inhibition/excitation balance (Albrecht et al., 2016). Within the ventral CA1 region, astrocytes contribute to enhanced long-term plasticity after juvenile stress by altering glutamate metabolism (Ivens et al., 2019).
Astroyctes provide a prime resource for long-term adaptation to stress, which we want to investigate also in future projects.